EMA Regulatory
Integral vs Co-packaged Drug-Device Combinations: The Classification Guide
Quick Answer
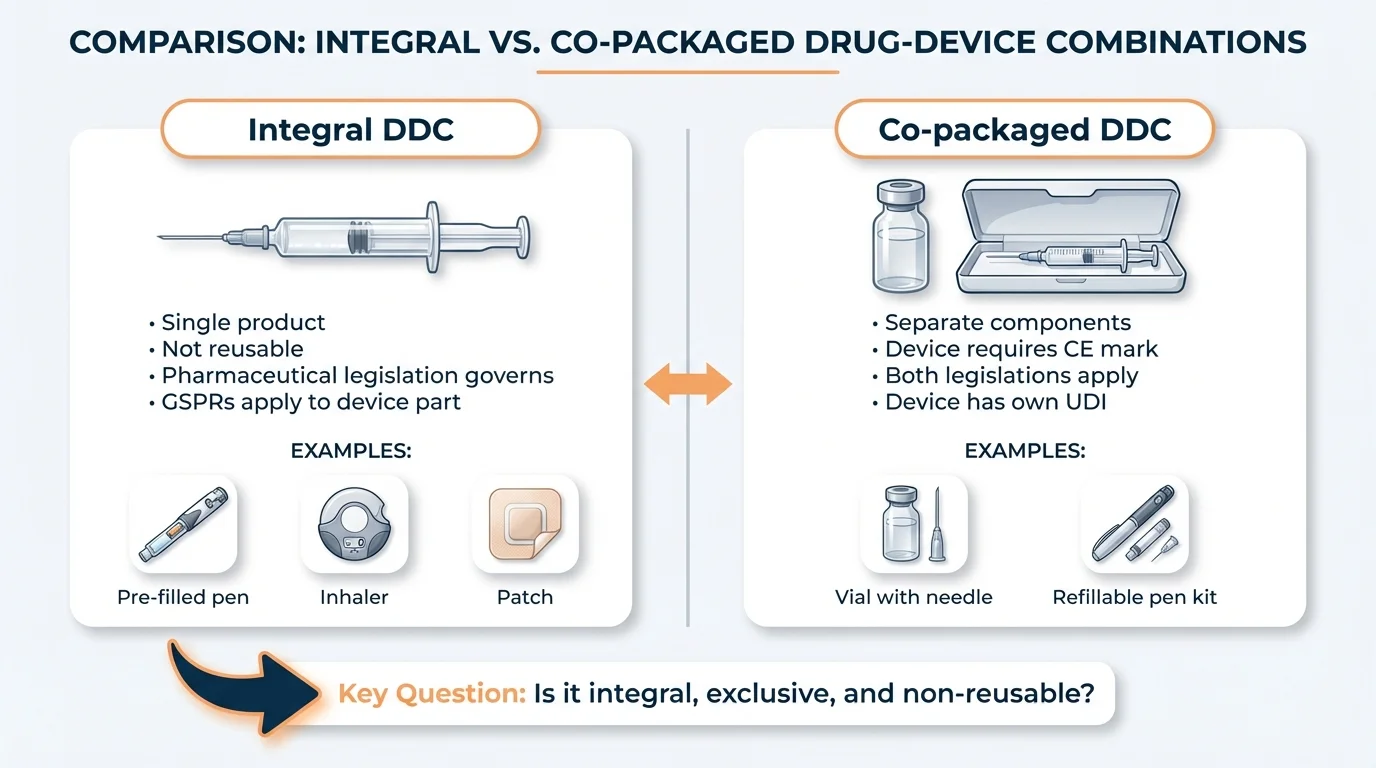
Integral drug-device combinations form a single, non-reusable product governed primarily by pharmaceutical legislation (Directive 2001/83/EC or Regulation 726/2004), with GSPRs applying to the device part. Co-packaged combinations contain separate products in the same packaging—the device component requires CE marking under MDR. The distinction turns on three cumulative conditions: single integral product, exclusive intended use, and non-reusability.
The quality director called at 7:30 AM. Her voice had that particular tension that meant something had gone sideways.
"The Notified Body says our pre-filled syringe kit needs CE marking."
"Which component?"
"The whole device. They're saying it's co-packaged, not integral."

The issue: their product included a pre-filled syringe with the drug solution, plus a separate reconstitution vial. The Notified Body considered the separate vial evidence that this wasn't a "single integral product"—it was two products in one box.
The distinction between integral and co-packaged isn't academic. It determines which legislation governs your product, whether you need CE marking, and how you label the final package. Getting it wrong means regulatory rework measured in months.
Regulatory Definitions: What Makes a Product Integral?
Why this matters: These definitions have legal force. The language in MDR Articles 1(8) and 1(9) creates the classification framework—understanding the exact wording prevents misinterpretation.
MDR Article 1(9) defines two scenarios for drug-device combinations involving administration devices:
Integral DDC (Article 1(9) Second Subparagraph)
When all three conditions are met:
- • Single integral product at time of placing on market
- • Intended exclusively for use in that combination
- • Not reusable
→ Governed by Directive 2001/83/EC or Reg. 726/2004
Co-packaged DDC (Article 1(9) First Subparagraph)
When any condition is NOT met:
- • Medicinal product and device in same secondary packaging
- • But NOT forming a single integral product
- • Device component separate and identifiable
→ Device falls under MDR; requires CE marking
Article 1(8) addresses a different scenario: devices that incorporate a medicinal substance as an integral part (like drug-eluting stents). Here, the key question is whether the substance's action is principal or ancillary—covered in detail in our classification rules guide.
The Three Cumulative Conditions for Integral Status
Why this matters: "Cumulative" means all three conditions must be satisfied. Missing any one routes your product to the co-packaged pathway—with its CE marking requirements.
The Three-Condition Test
- 1
Single Integral Product
The device and medicinal product form ONE product at the moment of placing on market—not assembled by the user
- 2
Exclusive Intended Use
The device is intended EXCLUSIVELY for use with that specific medicinal product combination—no alternative uses
- 3
Not Reusable
The device cannot be refilled, recharged, or used again with new medicinal product
Let's examine each condition in detail:
Condition 1: Single Integral Product
The device and medicinal product must be combined into one unit before market placement. This typically means factory assembly, not end-user assembly.
Examples That Meet This Condition
- • Pre-filled syringe with drug solution ready for injection
- • Pre-filled pen with cartridge already loaded
- • Transdermal patch with drug incorporated in matrix
- • Nasal spray with drug solution already in device
Examples That Typically Do NOT Meet This Condition
- • Vial of drug + empty syringe in same box (user assembles)
- • Lyophilized drug + diluent + syringe kit (reconstitution required)
- • Cartridge sold separately from injection pen (assembled by user)
Condition 2: Exclusive Intended Use
The device must be designed and intended solely for use with that specific medicinal product. If the device could be used with other products, this condition fails.
Exclusive vs Non-Exclusive Intended Use
| Scenario | Exclusive (Condition Met) | Not Exclusive (Condition NOT Met) |
|---|---|---|
| Pre-filled syringe | Factory-filled, labeled for specific drug only | Universal syringe compatible with multiple drugs |
| Injection pen | Pen designed only for proprietary cartridge format | Pen accepts standard cartridges from multiple manufacturers |
| Inhaler | Device designed for specific drug formulation only | Generic inhaler that accepts multiple drug capsules |
Condition 3: Not Reusable
The device cannot be refilled or used again after the medicinal product is exhausted. This is usually the clearest condition—either the device is disposable or it isn't.
Critical Distinction: Single-Use vs Reusable
A device that delivers multiple doses but is then discarded (like a 30-day supply inhaler) is NOT reusable for this purpose—it's designed for one treatment course. A device that can be refilled with new cartridges IS reusable, even if each cartridge is single-use.
Regulatory Pathways: Where Each Type Goes
Why this matters: The pathway determines which body assesses your product, what documentation you need, and how long the process takes. Integral and co-packaged products follow fundamentally different routes.
Regulatory Pathway Comparison
| Aspect | Integral DDC | Co-packaged DDC |
|---|---|---|
| Primary legislation | Directive 2001/83/EC or Reg. 726/2004 | MDR 2017/745 for device; Pharma for drug |
| Authorizing body | EMA or National Competent Authority | NB (device) + EMA/NCA (drug) |
| CE marking | NOT required for product | REQUIRED for device component |
| Device conformity | GSPRs via MA dossier (Article 117) | Full MDR conformity assessment |
| Documentation | Module 3.2.R in MA dossier | Device technical file + MA dossier |
| UDI | Not on outer packaging | Required on device/immediate packaging |
The Article 117 Mechanism: For integral DDCs, the marketing authorization dossier must address the device part. This requires including a Declaration of Conformity, CE certificate (if available), or Notified Body opinion confirming compliance with the relevant GSPRs of MDR Annex I.
Co-packaged: Both Frameworks Apply
For co-packaged products, you're effectively running two parallel regulatory processes: MDR conformity assessment for the device (leading to CE marking) and pharmaceutical authorization for the drug. The Product Information annexes of the medicinal product should NOT include device-specific administrative information (manufacturer, CE mark number, device UDI)—these belong on the device labeling.
Labeling Requirements: Critical Differences
Why this matters: Incorrect labeling can trigger recalls, even if the underlying product is correctly classified. The rules for what appears where are explicit—and different for each product type.
Integral DDC Labeling
Integral combinations follow pharmaceutical labeling requirements as specified in QRD templates. Key points:
Integral DDC Labeling Requirements
- Follow QRD template for medicinal product labeling
- UDI should NOT appear on outer packaging of the medicinal product
- If UDI is already directly marked on device part, it need not be removed
- Product Information follows Directive 2001/83/EC format
- No CE mark symbol on product (not applicable)
- Batch number and expiry date per pharmaceutical requirements
Co-packaged DDC Labeling
The device component in a co-packaged product must carry its own MDR-compliant labeling:
Co-packaged Device Labeling Requirements
- CE marking with Notified Body number (if applicable)
- Device identification and manufacturer identification
- Lot number or serial number
- UDI carrier on device or immediate packaging
- Authorized representative information (if applicable)
- Relevant symbols per MDR Annex I Section 23
Keep Device and Drug Information Separate
For co-packaged products, the medicinal product's SmPC, labeling, and package leaflet should follow Directive 2001/83/EC and must NOT include administrative information pertaining to the medical device. Device-specific information belongs on the device labeling, not mixed into pharmaceutical documentation.
Product Examples: Integral vs Co-packaged
Why this matters: Classification decisions are easier to understand through concrete examples. These are the products that regulatory professionals encounter regularly.
Integral Drug-Device Combinations
Administration Devices
- • Pre-filled syringes
- • Pre-filled pens and autoinjectors
- • Nebulisers pre-charged with specific medicinal product
- • Nasal and oromucosal sprays
- • Pre-filled inhalers (DPIs ready for use)
- • Pre-assembled, non-reusable vaginal applicators
Delivery Systems
- • Transdermal patches for drug delivery
- • Drug-releasing intrauterine devices
- • Implants containing medicinal products
- • Medicinal products with embedded sensors
- • Dropper tops on eye drop containers
- • Mouthpiece on throat spray cans
Co-packaged Drug-Device Combinations
Administration Accessories
- • Oral administration devices (cups, spoons, syringes)
- • Injection needles and filter needles
- • Spacers for inhalation sprays
- • Electronic tablet dispensers
- • Empty sterile syringe with vial
Reusable Devices
- • Refillable pens and injectors (using cartridges)
- • Reusable dry powder inhalers
- • Nebulisers (separate from medicinal product)
- • Vaporisers
- • Pumps for medicinal product delivery
Grey Areas and Edge Cases
Why this matters: Not every product fits neatly into integral or co-packaged. These edge cases are where classification disputes arise—and where early guidance is most valuable.
Grey Area 1: Reconstitution Kits
A lyophilized drug with reconstitution diluent and pre-filled syringe for administration—is the syringe integral or co-packaged?
Analysis: If the syringe is pre-filled with diluent and used to reconstitute AND administer the drug, it may qualify as integral (EMA has examples of such products). If the syringe is empty and used only for administration after separate reconstitution, it's likely co-packaged.
Grey Area 2: Multi-Use Pens with Disposable Cartridges
A reusable injection pen sold with a medicinal product cartridge—what's the classification?
Analysis: The pen is clearly reusable (fails condition 3), so it's co-packaged and requires CE marking. However, if a non-reusable pen is sold pre-loaded with a cartridge that cannot be replaced, the combination may be integral.
Grey Area 3: Connected Devices with Software
An autoinjector with embedded connectivity that transmits dose data to a smartphone app—integral or not?
Analysis: The connectivity function doesn't change the fundamental classification. If the physical device meets all three conditions, it's integral. The software component may have separate regulatory considerations under MDR (software as a medical device), but the combination product classification follows the same rules.
A Practical Assessment Framework
Step 1: Apply the Three-Condition Test
- Condition 1: Is the product a single unit at time of market placement? (Factory assembled, not user assembled)
- Condition 2: Is the device designed exclusively for this specific combination? (No alternative uses)
- Condition 3: Is the device non-reusable? (Cannot be refilled or used again)
- Result: If YES to all three → Likely integral. If NO to any → Likely co-packaged.
Step 2: Document Your Analysis
- • State each condition and how your product meets (or doesn't meet) it
- • Reference comparable products with established classifications
- • Address any ambiguities explicitly—don't leave them for assessors to question
- • Include design documentation supporting your analysis
Step 3: Seek Guidance for Edge Cases
- • Consult national competent authority for formal classification advice
- • Request EMA ITF meeting for informal scientific input
- • Review MDCG guidance documents on combination products
- • Test your classification approach before committing to a pathway
References
- 1. Regulation (EU) 2017/745 on medical devices (MDR), Articles 1(8), 1(9), 1(10)
- 2. Directive 2001/83/EC relating to medicinal products for human use
- 3. EMA Questions and answers on implementation of the MDR and IVDR (EMA/37991/2019 Rev.6)
- 4. EMA Draft Guideline on Quality Requirements for Drug-Device Combinations
- 5. MDCG 2019-2 Guidance on UDI rules for device-part of products referred to in Article 1(8), 1(9), 1(10)
Need faster answers? RegulatorySense delivers instant, authoritative guidance with source citations.
FAQ
What is the difference between integral and co-packaged drug-device combinations?
How do I determine if my product is integral or co-packaged?
Does an integral DDC need CE marking?
What labeling requirements apply to co-packaged vs integral combinations?
Is Your DDC Integral or Co-packaged?
The three-condition test sounds simple. The edge cases aren't. Two minutes to check your classification logic.
Stop searching through hundreds of PDFs. Get authoritative answers in seconds.
Validate Your Approach →5 questions · Personalized insights · Free